People
- Department of Physiology
- About Physiology and Biophysics
-
Education
- Physiology and Biophysics Educational Programs Home
- Graduate Program
- Undergraduate Research Experience
- Seminar and Lecture Series
- Seminar Series Archives
-
Research
- Physiology and Biophysics Research Home
- Core Facilities
- Programs
- Graduate Program Research
- Publications
- Research Links
- Resources
Li Research Summary
We primarily seek to understand the intrinsic relationship between diabetes, cardiovascular diseases, and neurodegenerative disease. Evidence suggests that adenosine monophosphate-activated protein kinase (AMPK) is a critical metabolic sensor to maintain metabolic homeostasis and inflammatory response under physiological and pathological conditions. We want to elucidate the molecular mechanisms responsible for AMPK activation, identify novel downstream AMPK targets, and develop therapeutic techniques that target the enzyme to prevent and treat myocardial ischemia, hypertension, cardiac hypertrophy, diabetes, and Alzheimer’s disease related dementia.
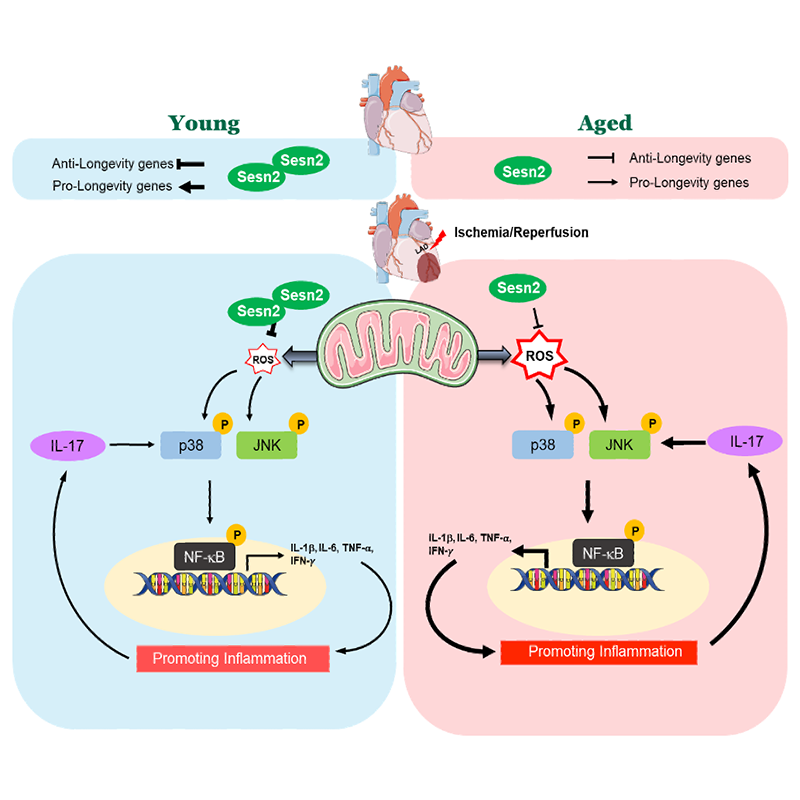
Sesntrin2 modulates AMPK signaling: Ischemia heart disease is a leading cause of death world-widely and has increased prevalence and exacerbated myocardial infarction with aging. Sestrin2 (Sesn2), a stress-inducible protein, declines with aging in the heart and the rescue of Sesn2 in the aged mouse heart improves the resistance to ischemic insults caused by ischemia and reperfusion. Through a combination of transcriptomic, physiological, histological, and biochemical strategies, we unveiled that Sesn2 deficiency shows an aged-like phenotype in the heart with excessive oxidative stress and provoked immune response. While challenged with ischemia and reperfusion stress, the transcriptomic alterations in Sesn2 knockout mouse heart resembled aged wild type mouse heart. Sesn2 plays a crucial role in modulating inflammatory response through maintaining the intracellular redox homeostasis in the heart under ischemia reperfusion stress condition. Sesn2 could be a potential target for treatment of age-related ischemic heart disease.
Go here for the image long description.
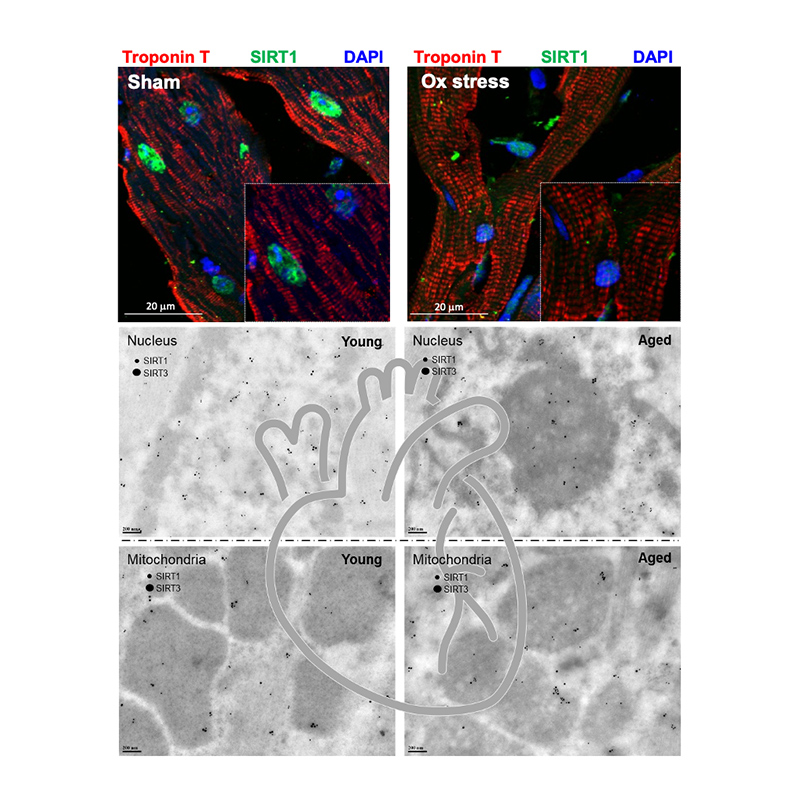
Sirtuin signaling in cardiac metabolism: Sirtuin family members protect cardiomyocyte function against ischemia and reperfusion injury. C57BL/6J wild type mice, inducible cardiomyocyte-specific SIRT1 knockout (icSIRT1-/-), and cardiomyocyte-specific SIRT3 knockout (cSIRT3-/-) mice are subjected to isolate cardiomyocytes. The deletion of SIRT1 or SIRT3 in cardiomyocytes impairs cardiac contractility during ischemic stress. The biochemical and seahorse analysis showed that the deficiency of SIRT1/SIRT3 in aging leads to the impaired activation of AMPK and alterations in mitochondrial oxidative phosphorylation (OXPHOS) that causes impaired mitochondrial respiration in response to stress stimuli.
 Go here for the image long description.
Go here for the image long description.
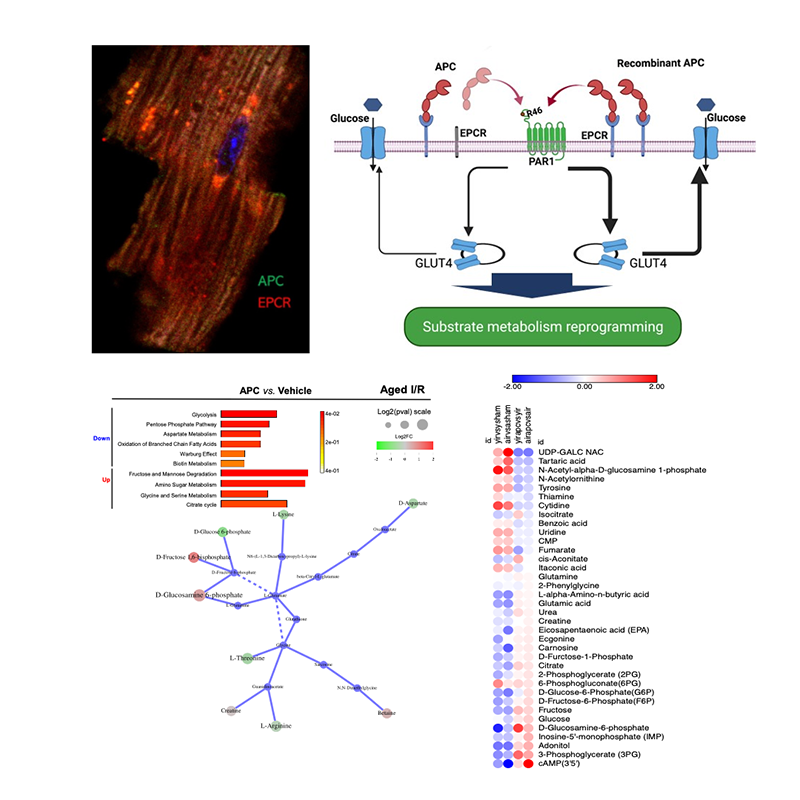
APC signal transduction in acute injury: Activated protein C (APC) is a plasma serine protease with anticoagulant and anti-inflammatory activities. Endothelial protein C receptor (EPCR) mediates APC's downstream anti-inflammatory effects. We have found that APC exerts cardioprotective effects during ischemia and reperfusion. APC, signaling-selective APC-2Cys, or anticoagulant-selective APC-E170A were bioengineer generated. Serum EPCR measurement showed that membrane EPCR's shedding into circulation was dramatically promoted during ischemia and reperfusion stress. Administration of the recombinant APC reduced EPCR shedding caused by ischemic stress in wild type but not in EPCRR84A/R84A heart. The metabolomics analysis and working perfusion heart results demonstrated that AMP-activated protein kinase (AMPK) signaling mediates acute adaptive metabolic response while protein kinase B signaling pathway is involved in chronic metabolic programming in the hearts treated with APC derivatives. Therefore, administration of APC derivative could be a strategy for limiting cardiac damage in aging.
Go here for the image long description.
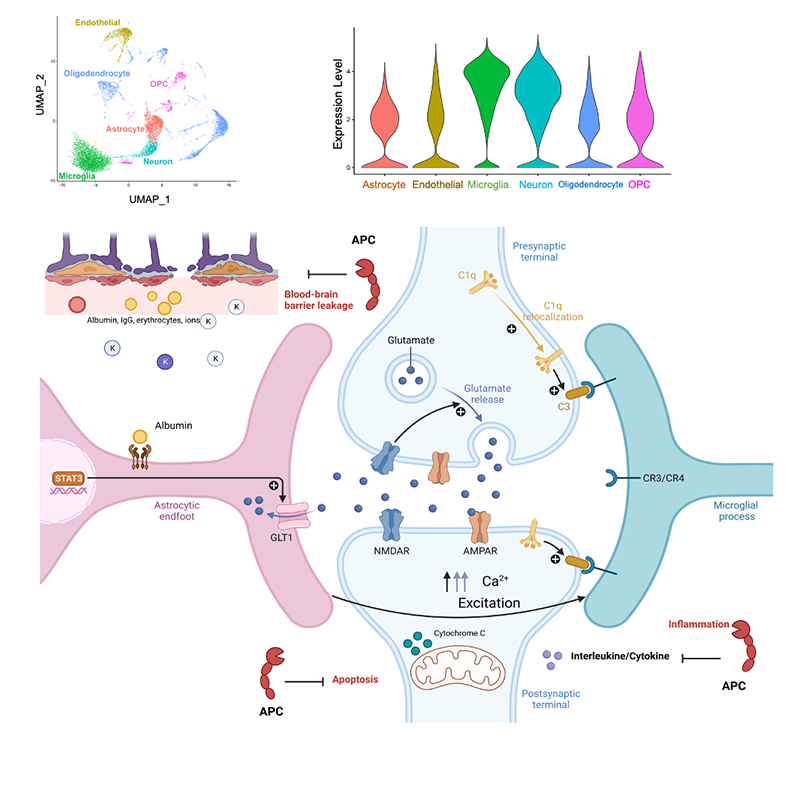
APC signaling in neurodegenerative diseases: Aging is among the most important risk factors for Alzheimer's disease (AD). APC is a plasma protease exerting cytoprotective effects, including modulating metabolic pathways, repressing inflammation, and protecting neurons from stroke and trauma. ScRNA-seq allows for examining the underlying mechanisms by which APC therapy on AD at the cellular level. Bioinformatic analysis of AD animal model of 5XFAD (Swedish K670N, M671L, Florida I716V, London V717I, and two mutations in the human presenilin-1 gene: M146L and L286V) versus littermate wild type (WT) C57BL/6J brain revealed much of the sample consists of microglial cells. Interestingly, 5XFAD versus WT brains have notable decreases in astrocyte and neuron populations. Administration of recombinant APC rebounds these populations to comparable levels as WT while dramatically reduces the microglial population in 5XFAD versus WT. Differential expression testing conveys transcriptomic regulation of known AD critical genes such as Apoe, Ctsb, Trem2, and Tyrobp by APC. Consequently, GO term enrichment uncovers inflammatory biological processes such as microglial activation to be downregulated by APC-treated 5XFAD. Our long-term objective is to understand mechanisms of APC in AD pathogenesis to develop an effective therapeutic strategy for the AD patients.